Details of the Drug
General Information of Drug (ID: DMJ7A0H)
| Drug Name |
Cefdinir
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CFDN; Cefdinirum; Cefdinyl; Cefdirnir; Cefzon; Omnicef; BMY 28488; FK 482; PD 134393; Cefdinir [USAN:INN]; Cefdinirum [INN-Latin]; Cefzon (TN); FK-482; FR-80482; KS-1038; Omnicef (TN); PD-134393; Cefdinir (JP15/USAN/INN); Omnicef, FK-482, BMY-28488, PD 134393, CI-983, Cefdinir; (-)-(6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7(sup 2)-(Z)-oxime; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-hydroxyiminoacetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(hydroxyimino)acetyl]amino}-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-(2 (2-aminothiazol-4-yl)-2-hydroxyiminoacetamido)-3-vinyl-3-cephem-4-carboxylic acid; 7beta-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(hydroxyimino)acetamido]-3-ethenyl-3,4-didehydrocepham-4-carboxylic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
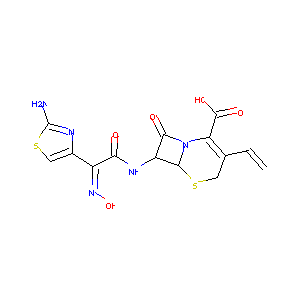 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 395.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cefdinir (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Emerging therapies for the treatment and prevention of otitis media. Expert Opin Emerg Drugs. 2006 May;11(2):251-64. | ||||
|---|---|---|---|---|---|
| 2 | Cefdinir FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | FDA Approved Drug Products: Omnicef (cefdinir) | ||||
| 5 | DailyMed: Cefdinir oral capsules | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Perry CM, Scott LJ: Cefdinir: a review of its use in the management of mild-to-moderate bacterial infections. Drugs. 2004;64(13):1433-64. doi: 10.2165/00003495-200464130-00004. | ||||
| 8 | Decreased affinity of mosaic-structure recombinant penicillin-binding protein 2 for oral cephalosporins in Neisseria gonorrhoeae. J Antimicrob Chemother. 2007 Jul;60(1):54-60. | ||||
| 9 | Transporter-mediated drug delivery: recent progress and experimental approaches. Drug Discov Today. 2004 Aug 15;9(16):712-20. | ||||
| 10 | Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol. 2005 Oct 1;70(7):1104-13. | ||||
| 11 | Barnett ML "Inhibition of oral contraceptive effectiveness by concurrent antibiotic administration." J Periodontol 56 (1985): 18-20. [PMID: 3882930] | ||||
| 12 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 13 | Chrysos G, Gargalianos P, Lelekis M, Stefanou J, Kosmidis J "Pharmacokinetic interactions of ceftazidime and frusemide." J Chemother 7 Suppl (1995): 107-10. [PMID: 8904125] | ||||
| 14 | Brown G, Zemcov SJ, Clarke AM "Effect of probenecid on cefazolin serum concentrations." J Antimicrob Chemother 31 (1993): 1009-11. [PMID: 8360120] | ||||
| 15 | Product Information. Omnicef (cefdinir). Parke-Davis, Morris Plains, NJ. | ||||
